6.02 X 10^23
Step 2 2 of 3. In either case what this is really saying is that you move.

Mole Concept Powerpoint Slides
Web So they started by defining.

. Web Answer 1 of 4. Convert to Regular Notation 6021023. The given problem will solve by using Avogadro number.
A mole of a substance can be defined as. This is a chemistry geek day. Web To be specific at 602 is the precise time at which we celebrate Mole Day ideally in a chemistry lab or classroom somewhere.
If you were to write this in a calculator it would be 602E23. So if there are 60221023. To the nearest orderof magnitude how many moles of atoms are in a large domesticcat.
602 1023 602 10 23. It refers to the amount of. It is the number of atoms ions and molecules in one gram atom of element one gram molecules.
The masses of a hydrogen atom. The amount of substance that contains 6021023. Web Approximately 5981013 meters.
Web One mole of one substance contains 602 x 10 23 textbf23 23 atoms or molecules. The number of atoms in a 12g sample of C-12 B. Web Answer 1 of 8.
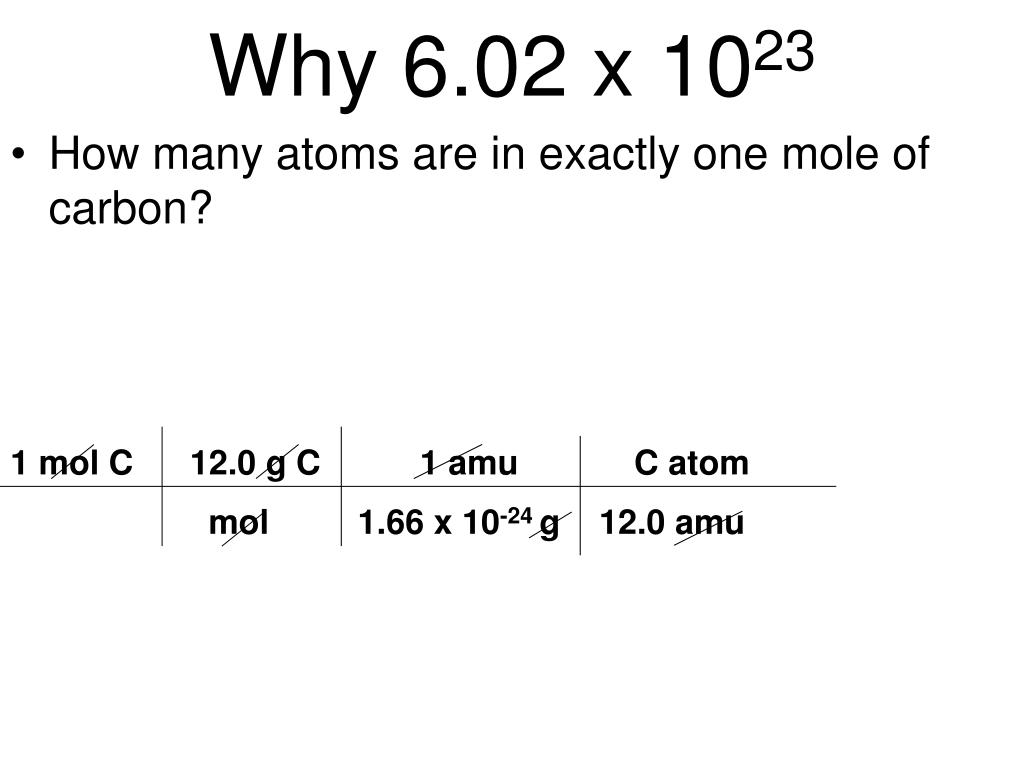
Avogadro number connects amu. If I have 12 grams of carbon-12 not any other isotopes of carbon then it would have exactly Avogadros number of atoms in it. λ f c where.
The wavelength equation for an electromagnetic wave photons is. A mole just tells how many just like and. This is another way of writing scientific notation.
This number is called the Avogadro number. Web 10²³ 100000000000000000000000 23 zeroes 60223602100000000000000000000000. One mole represents 60221023 separate entities just like one dozen represents 12 objects.
Since the exponent of the scientific notation is positive move the decimal point 23 23 places to the. Web A mole of atoms is 602 x 1023 atoms. Web You have one mole of H_2O molecules.
The energy required to break one. We can write this number as. The SI value of a chemical entity such as atoms electrons or protons is known as the mole abbreviated mol.
Web Provided to YouTube by DistroKid602 x 1023 Bop LouieRISING SUN VOLUME ONE GoobGeeb RecordsReleased on. Web For example 10 3 would have the kilo prefix 10 6 would have the mega prefix and 10 9 would have the giga prefix. Web Why is the number 6022 x 10 23.
Web Therefore 602 x 10²³ seconds 602 x 10²³ seconds 315360000 seconds 1909 x 10⁸ x 10²³ 1909 x 10¹⁵ decades. Web 602 grams sulfur 1mole S3207 grams6022 X 10231 mole S 113 X 1023 atoms sulfur How many atoms are in a sulfur molecule that has the elemental.

Ch10 1 The Mole 1 Mol 6 02 X 1023 Particles Ppt Video Online Download

Chemistry Mole Sample Kit Purchase A Mole Element Project For Your Chemistry Class Experiments At Teachersource Com
Star Moles Episode 6 02 X 10 23 Schooltube Safe Video Sharing And Management For K12
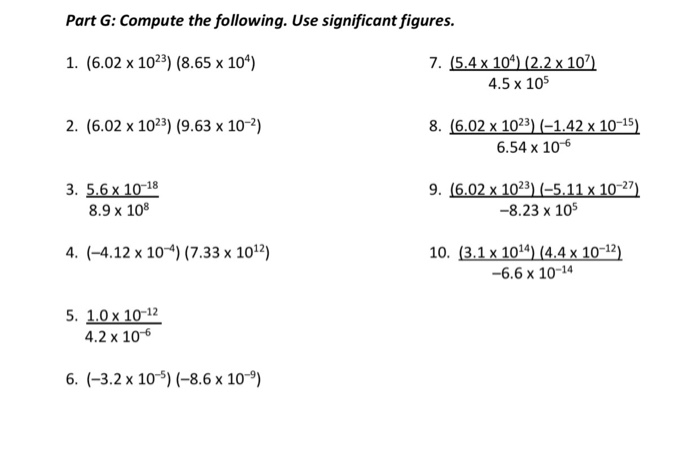
Solved Part G Compute The Following Use Significant Chegg Com
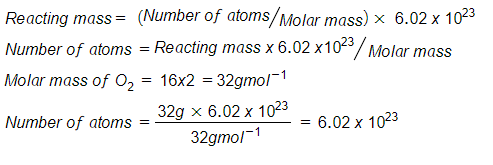
Introduction To The Mole Concept Classnotes Ng
Mole Day Celebration Chemistry Biochemistry Swarthmore College

One Mole Of Co2 Contains Chemistry Questions

Keep Calm And 6 02x10 23 On Poster Ormy Keep Calm O Matic

The Number Of Significant Figures In 6 02xx10 23 Is Youtube
The Mole
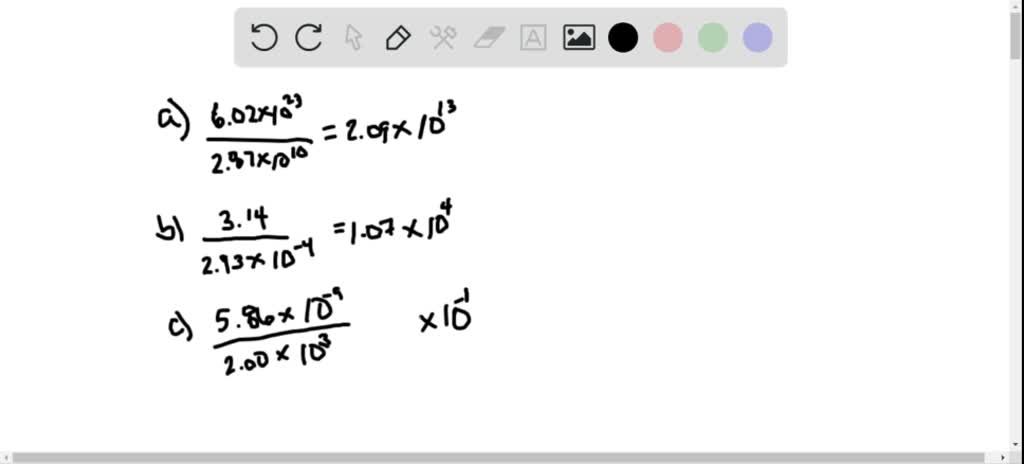
Solved Divide A 6 02 10 23 2 87 10 10 B 3 14 2 93 10 4 C 5 86 10 9 2 00 10 3 D 7 8 10 12 9 3 10 14 E 6 83 10 12 5 02 10 14
Ppt Chapter 8 The Mole Part 1 Powerpoint Presentation Free Download Id 5435580

Avogadro S Constant Surfguppy Chemistry Made Easy For Visual Learners

6 02xx10 23 Molecules Of Urea Are Present In 100 Ml Of Its Solution The Concentration O Youtube

Avogadro S Number The Mole Grams Atoms Molar Mass Calculations Introduction Youtube
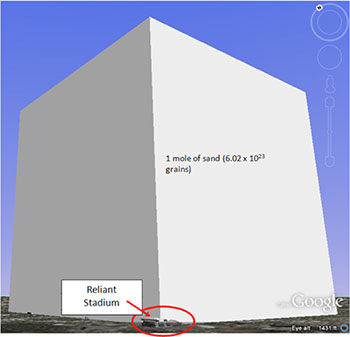
Untitled Document

Solved A Mole Of Sodium Atoms Contains 6 02 X 10 23 Atoms How Many Moles Would Be Needed In Order To Have 2 50 X 10 24 Atoms